Search
Pet Food Factory Explosion – A Lesson in Combustible Dust
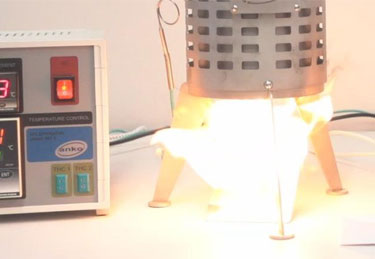
It was the neighbors that first noticed the problem.
Think Italy. Think a small, provincial town in Lombardy in the north; an area that would eventually be badly struck by the devastating Coronavirus pandemic.
On April 1st, 2 years ago, residents near a pet food factory became aware of an unusual odor – a ‘miasma’ (ref. 1). Three technicians were sent to the plant to investigate. Two of them would never return home.
The trio quickly identified the source of the problem. It seemed to be associated with a flour drying facility which, according to reports (ref. 1), had been shut down for 4 days. They established that the odor was coming from inside one of the dryers. They set out to see what could be done. Two of the technicians climbed onto a platform adjacent to the dryer. There was an explosion which apparently killed the 2 technicians on the spot.
At the time of reporting, investigations were ongoing, but it was surmised that it was an ‘out of control chemical reaction’ which was at the root of the problem.
Readers of earlier editions of our Safety Dispatches may recall another incident on which we reported a few months ago (ref. 2) where a combustible dust explosion took more than a day to develop. That time it was wood dust that smoldered. The Lombardy explosion here involved flour used in pet food manufacture.
Flour, like wood dust, can exhibit a tendency to self-heat if circumstances contrive to encourage this. Self-heating (and eventually spontaneous combustion) is caused by the exothermic gas-solid reaction of oxygen molecules on the surface of each grain/grain dust particle. At ambient temperatures, these reactions proceed across grain/dust surfaces, but heat is produced throughout the bulk of any deposits since air is inevitably trapped between the particles. Self-ignition will occur when the heat production rate within the bulk or layers is greater than the heat dissipation rate and the temperature has reached a certain minimum value. The reaction speed increases with temperature, so whereas it can take a long time for significantly raised temperatures to develop, once the reaction has started it will speed up over time; faster and faster. The more oxygen molecules react with each solid grain/dust particle within a certain time interval, the greater the rate of combustion will be.
Take a look at the charts below to see what thermal runaway looks like in practice.
These charts display results obtained in our test laboratories on a carbonaceous material placed in a wire basket at the center of a temperature-controlled oven.
Chart 1: Isothermal test in a 100mm3 basket @ 120°C
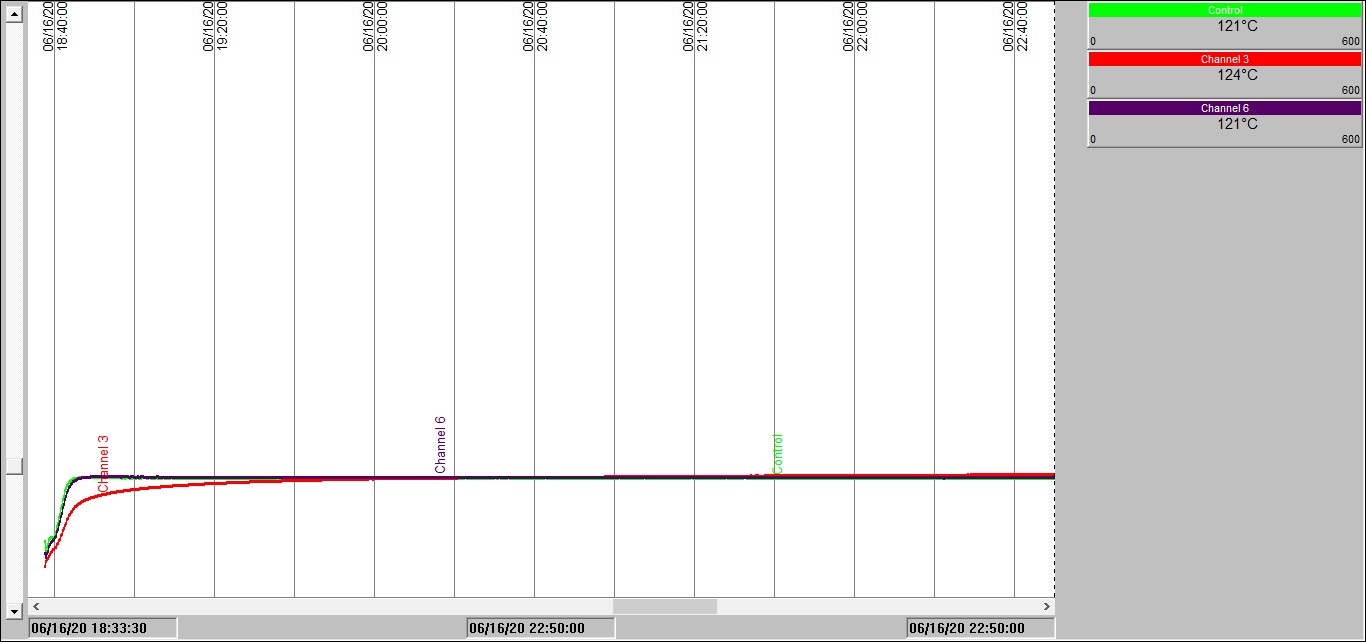
Chart 1 shows temperature traces over time measured inside one of our ovens held at 120°C. The red trace is measuring the temperature inside a 100mm cubic sample of the carbonaceous material located at the center of the oven. The green and blue traces show internal oven temperature measurements. Note how, unsurprisingly, it takes a little while for the sample temperature to reach the oven temperature; we have to wait for it to be heated-up by conduction – just as in your oven at home.
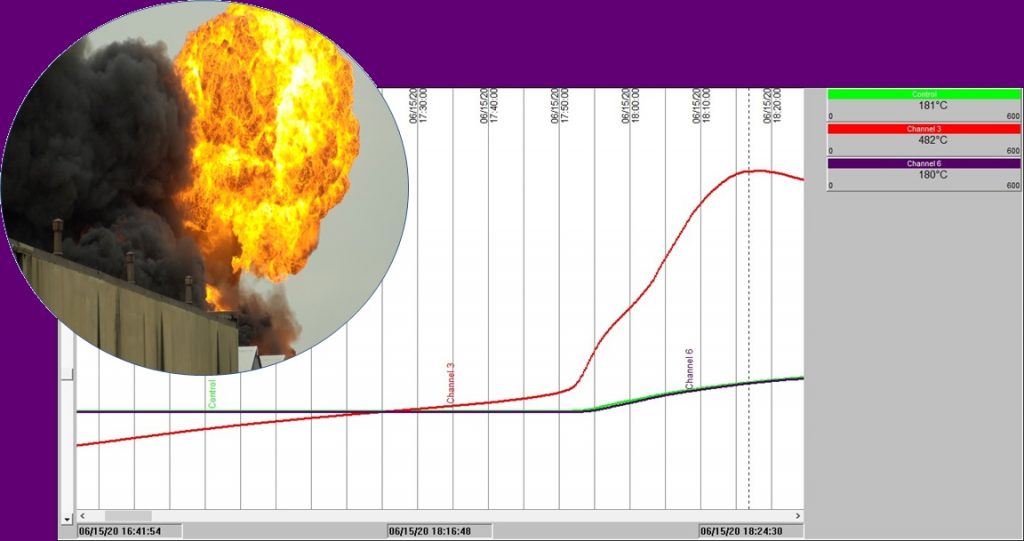
Chart 2: Isothermal test in a 100mm3 basket @ 140°C
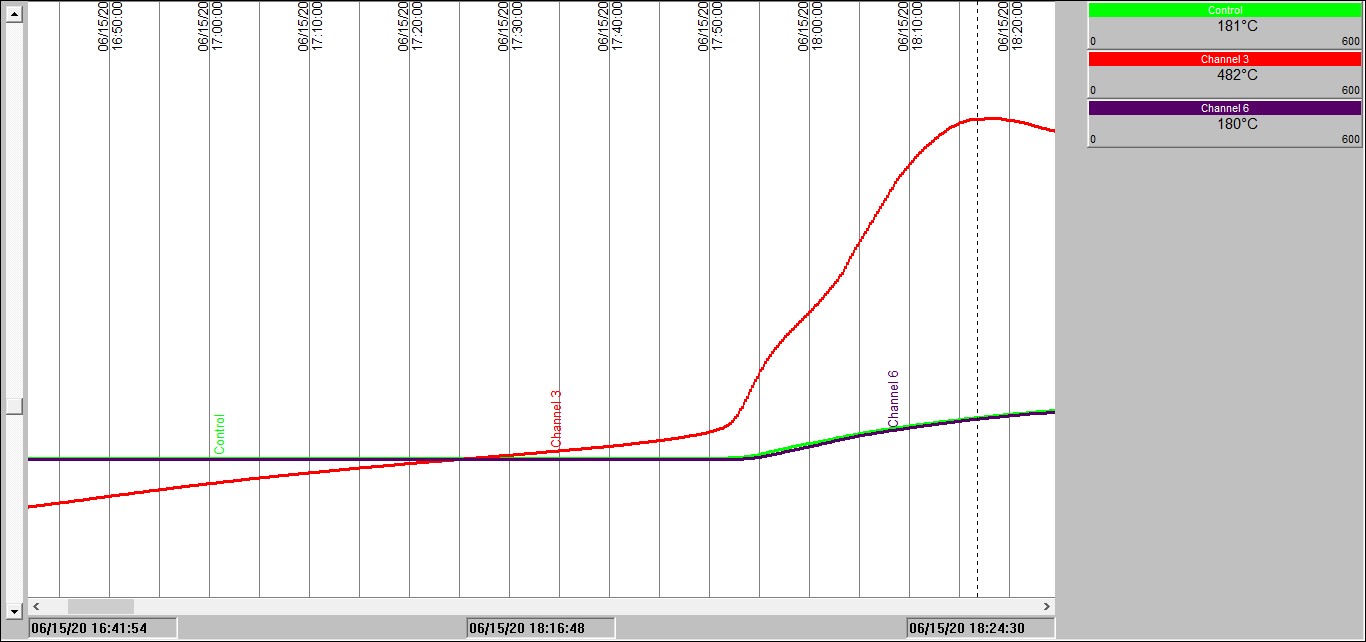
Chart 2 shows exactly the same experiment as with Chart 1, except this time the oven temperature was fixed initially at 140°C. This time we see that after an hour and a half or so the sample temperature rises slowly ABOVE the oven temperature. 30 minutes later, all hell breaks loose! Sample temperature rockets up. The combustion that takes place is severe enough to not only increase the sample temperature to approaching 500°C, but the burning sample then starts to increase the temperature inside the oven space – see the blue and green temperature traces rise.
These traces we obtained are not for the flour involved in the Lombardy explosion – but they serve to illustrate very well the problem of self-heating leading to spontaneous combustion. Slow smoldering combustion – sometimes over hours or days, followed by rapid temperature rise and fire. In fact, what can happen if someone happens along and opens an inspection hatch, for example, oxygen rushes in and increases the combustion rate – and can even disperse dust to create a full-on combustible dust explosion.
There are a number of techniques available to warn against such thermal runaway scenarios but in our view, everything should begin with DATA. Knowledge on the self-heating, combustion and explosion characteristics of your product can be used to establish the conditions under which smoldering can begin – and can ensure that plant is properly designed such that if an explosion were to occur, people and plant are protected.
If you would like to learn more about spontaneous combustion and self-heating and how it can be controlled on your facility, please contact us at 609-455-0001 or email us at [email protected].
Ref 1: newsbeezer.com, April 1st 2018
Ref 2: https://stonehousesafety.com/ss-case-studies/the-delayed-dust-explosion-in-belgium/
Ref 3: Seitz S., Franke J., Giebisch G., 2016, Determination of the self-ignition behavior of bulk materials from heat storage tests below atmospheric pressure, Chemical Engineering Transactions, 48, 583-588 DOI:10.3303/CET1648098

Get in touch
To learn more about our expertise and services in dust explosion prevention & mitigation, call us at +1 609 455 0001 or email us at [email protected] today.
We also offer tailored virtual and in-company process safety training programs on Dust Explosions, Static Electricity and HAC (Hazardous Area Classification) and more. Find further information here.