Two Explosions, Two Fires, Two Days, No Water
April 4th, 2020 was a normal Saturday at a specialist cored-wire manufacturing plant in Plum, Pennsylvania. The day’s routine was abruptly shattered by an explosion and fire in a production machine handling titanium and calcium powders (ref. 1). Three people were hospitalized. The fire service attended and extinguished the fire. The Fire Chief attending the scene remarked that he was grateful that the water fire extinguishing sprinkler systems DID NOT operate.
Meanwhile, 1 day later in the Hannover region of Germany, the local fire service was in attendance at a metal recycling facility (ref. 2). An explosion, a fire, and one worker hospitalized. Again, the attending fire service made a point of NOT using water to extinguish the fire.
So, let’s look at the problems with metal powders, why they can be potentially so dangerous, and what to do to reduce the risk …
Metal powders can be generated in many industrial processes including grinding, cutting, polishing, and buffing; and also, in the cases described above, in cored cable manufacture and metal recycling. Metal powders can also be produced deliberately in spray coating operations – another process that has led to a number of industrial explosions over the years.
The Fire and Explosion Hazards
With metal powders, from a process safety point of view, one of our concerns often is the chemical reaction that takes place between metal and water or water vapor. Specifically, a metal can react with water in an oxidation process that can result in the formation of a metal hydroxide, hydrogen gas, and a lot of heat (exothermicity). There are also some metals that can exhibit pyrophoric behaviour (spontaneous ignition in contact with oxygen/air). In these 2 cases, the process safety issues then become apparent; a high temperature reaction leading to fire (or glowing heaps of metal powder), hydrogen production (one of the most strongly explosive gases we know), and spontaneous ignition through pyrophoric behaviour.
But there is more. If the powder is dispersed in air, a combustible dust explosion risk can exist. Indeed, in a dispersed metal dust cloud scenario, the high combustion reaction temperatures support explosion development through radiative heat transfer with the consequence that some of the most devastating dust explosions experienced by industry have involved metal powders. And as if that wasn’t enough, we then get to the risk of spark (ignition source) production through the thermite reaction involving the presence of a metal oxide and a fuel – (e.g. aluminum impacting with rust).
And we haven’t even gotten on to the risk of electrically conductive metal fines entering electrical equipment, producing short circuit and again, ignition risk.
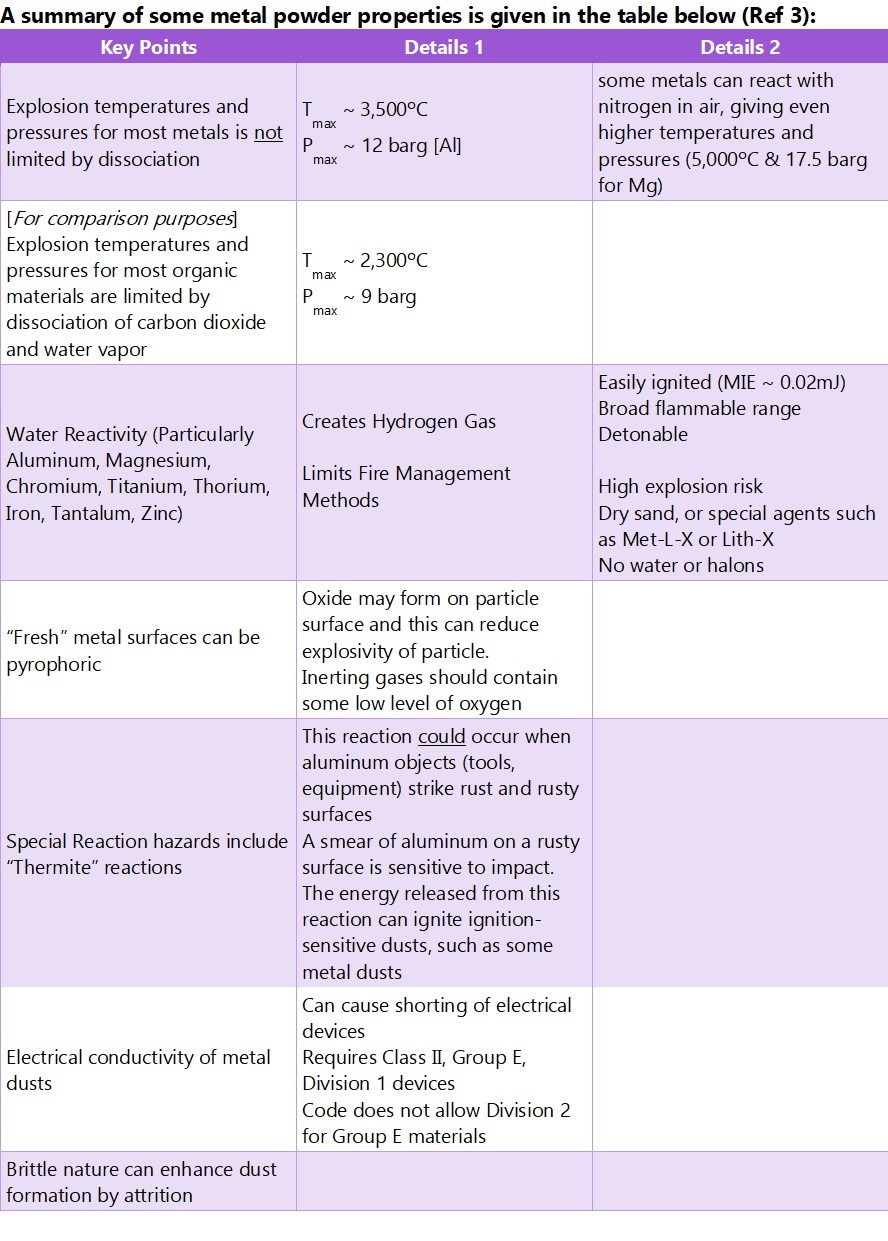
Note that it is metal fines that present one of the biggest hazards here because the oxidation that leads to fire and explosion risk takes place at surfaces; the interface between metal particles and water vapor, oxygen, etc. Powders have substantially more surface area per unit weight than chunks of metal! Surface oxidation of some metal powders will take place in air leading to an oxide coating of dust particles, but this is not always sufficient to stop a fire or explosion taking hold where conditions permit.
It must be said that not all metals are combustible or explosible; examples of combustible metals include aluminum, iron, magnesium, steel, titanium, and zinc. There are others.
So, it becomes clear why, in both the above reported explosions, there was some relief that water sprinkler systems did not activate. It’s all about avoiding the metal/water reaction process and more.
Clearly all processes that involve metal powders must be subject to detailed Process Hazard Analysis (PHA). Design to prevent hydrogen generation, dust explosion conditions, fire, pyrophoric activity, and thermite reaction is always the starting point. Explosion protection will also be needed as will separation of people from the processes. Electrically conductive dusts will also likely require appropriate electrical equipment selected after hazardous area classification exercise. Remember too, that hydrogen is a very light gas and could collect in the roof space should it be generated, so precautions are required to prevent hydrogen collection up there. Avoiding thermite reaction by avoiding impacts has to be considered and for some metals we need to prepare for risk of spontaneous ignition (pyrophoricity).
There is also the issue of providing training for all operators. You do not want a situation where a metal powder fire is made worse by an employee trying to use water – or reaching for a CO2 extinguisher, for example. Fire extinguishers used on many powders can quite easily CAUSE a dust explosion as they lift and disperse unburned powder into the metal fire. Class D fire extinguishers provide the key, so long as they do not cause dust to be raised. Sometimes fire-fighters will extinguish metal fires by gently smothering with sand. And of course, the local fire service needs to be aware of the nature of products used/stored on your facility – and how to respond in the event of incident.
Metal powder fires and explosions occur regularly in industry. If you would like assistance from one of our specialist consultants to advise on your Process Hazard Analysis (PHA), please contact us by emailing us at info@stonehousesafety.com or calling us on 609-455-0001.
Ref 1: https://triblive.com/local/pittsburgh-allegheny/3-injured-in-fire-at-plum-manufacturing-facility/
Ref 2: https://www.newslocker.com/de-de/region/bissendorf/bissendorf-feuer-in-recyclingbetrieb-ndrde/view/
Ref 3: Perry’s “Chemical Engineers’ Handbook”, 4th Ed.